Extended intra-nasal insulin (INI) treatment in an insulin-resistant early Mild Cognitive Impairment (MCI) patient improved grey matter volume, reduced beta-amyloid levels, and enhanced cognitive and pragmatic capacities, suggesting potential to slow neurodegenerative disease progression.
Highlights:
- Grey Matter Volume: After 9 months of INI treatment, the patient experienced an increase in grey matter volume.
- Beta-Amyloid Levels: INI treatment significantly lowered beta-amyloid levels in the patient’s brain.
- Cognitive Improvements: The patient’s scores on MCI and FAS tests improved, indicating better memory and executive functioning.
- Pragmatic Abilities: There was an observed increase in the patient’s pragmatic capacities, including improved social conversation and procedural memory.
- Clinical Alignment: These findings are consistent with prior clinical trials on INI, supporting its efficacy in improving cognitive and social functions in early MCI patients.
Source: Frontiers in Psychiatry (2024)
Major Findings: Intranasal Insulin Reverses Aspects of Mild Cognitive Impairment (2024 Case)
1. Increase in Grey Matter Volume
After nine months of intra-nasal insulin (INI) treatment, the patient, identified as EJ, showed a significant increase in grey matter volume.
Grey matter is crucial for processing information in the brain, encompassing regions involved in muscle control, sensory perception, memory, emotions, speech, decision making, and self-control.
The increase in grey matter volume suggests that INI treatment may help restore some of the brain tissue lost due to insulin resistance and early-stage Mild Cognitive Impairment (MCI).
This finding is particularly important because grey matter reduction is commonly associated with the progression of neurodegenerative diseases like Alzheimer’s.
2. Reduction in Beta-Amyloid Levels
The study found that INI treatment effectively lowered beta-amyloid levels in EJ’s brain.
Beta-amyloid is a protein that can form plaques, which are one of the hallmark signs of Alzheimer’s disease.
High levels of beta-amyloid are linked to brain cell damage and cognitive decline.
By reducing these levels, INI treatment may help mitigate the neurodegenerative processes associated with MCI and Alzheimer’s, potentially slowing down or even halting disease progression.
3. Cognitive Improvements
EJ’s cognitive abilities saw marked improvements over the course of the INI treatment.
Specifically, his scores on the MCI screen and FAS tests, which measure cognitive functions such as memory, attention, and verbal fluency, improved.
These enhancements indicate that INI treatment can positively affect various cognitive domains, contributing to better overall brain function.
Improved cognitive abilities can lead to a higher quality of life, enabling patients to perform daily tasks more effectively and maintain greater independence.
4. Enhanced Pragmatic Abilities
Pragmatic abilities, which refer to the use of language in social contexts, were also notably improved in EJ.
Before treatment, EJ experienced significant social and linguistic withdrawal, characterized by poor short-term memory, irritability, and reduced engagement in conversations.
After INI treatment, EJ showed increased capacities in social conversation and procedural memory.
He became more engaged in interactions, used humor and sarcasm appropriately, and displayed greater empathy and self-awareness.
These improvements suggest that INI treatment can help restore vital social skills that are often impaired in patients with MCI and Alzheimer’s, reducing social isolation and improving overall communication.
5. Alignment with Previous Clinical Trials
The findings of this study are consistent with results from previous clinical trials on INI treatment, which have also demonstrated cognitive and social benefits for patients with MCI and early Alzheimer’s disease.
These studies have shown that INI can improve memory, attention, reasoning, and language abilities, as well as enhance emotional self-awareness and reduce caregiver stress.
The alignment of this study’s results with earlier research reinforces the potential of INI as a viable treatment option for slowing the progression of neurodegenerative diseases.
Case Study: Intranasal Insulin for Cognitive Impairment (2024)
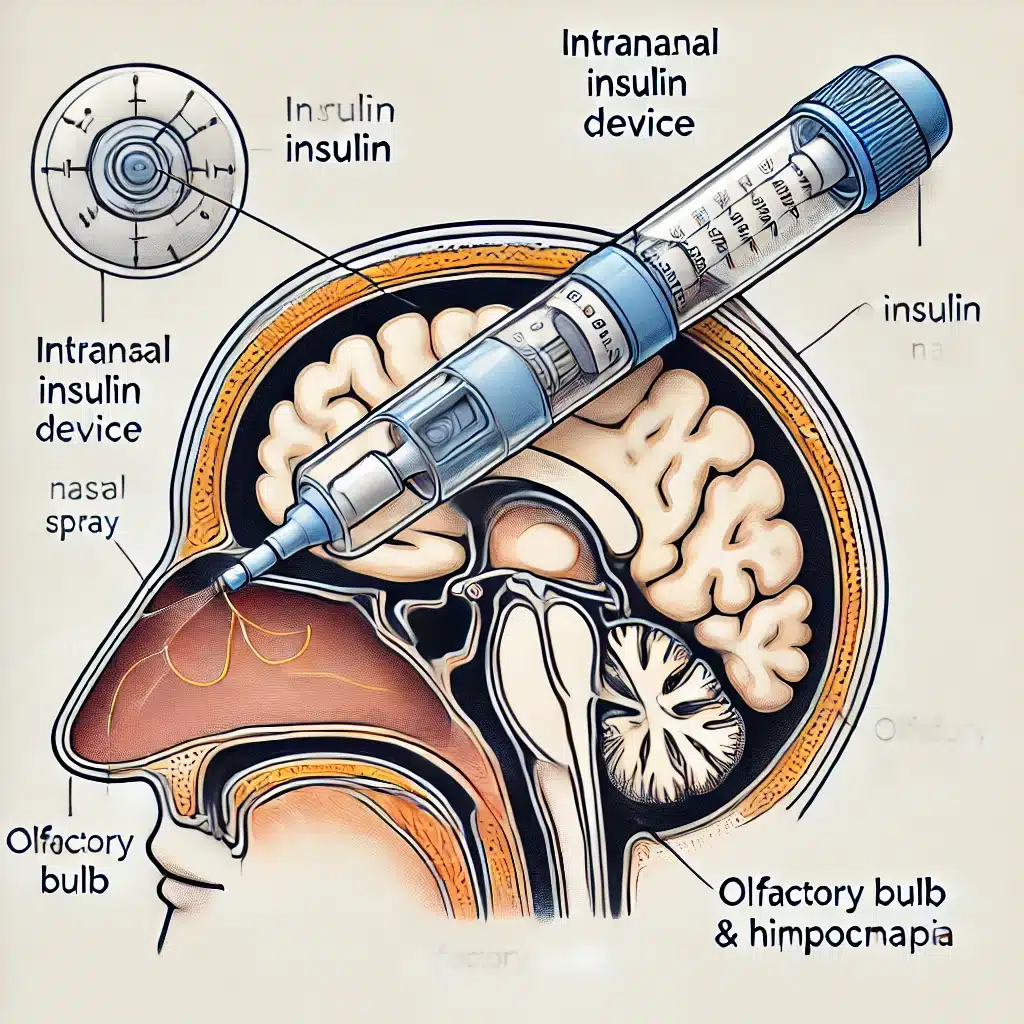
The aim of the study was to examine the effects of extended intra-nasal insulin (INI) treatment on a patient with insulin-resistant early Mild Cognitive Impairment (MCI), focusing on changes in grey matter volume, beta-amyloid levels, cognitive functions, and pragmatic abilities.
- Patient: One male patient, identified as EJ, aged 80, with a history of insulin resistance, hyperglycemia, medial temporal lobe (MTL) damage, and MCI.
- Background: EJ exhibited poor short-term memory, significant irritability, and social and linguistic withdrawal at the start of the treatment.
Methods
- Treatment: EJ received 40 IU of human insulin administered intra-nasally twice daily using a ViaNase device.
- Duration: The treatment lasted for nine months.
- MRI Scans: Conducted at baseline and after nine months to measure grey matter volume.
- Cognitive Tests: MCI screen and FAS test of phonemic verbal fluency were used to evaluate cognitive functions.
- Pragmatic Abilities: Assessed through observations, interviews, and audio recordings of communicative interactions.
- Beta-Amyloid Levels: Measured using cerebrospinal fluid biomarkers.
Limitations
- Sample Size: The study involved only one patient, limiting the generalizability of the findings.
- Duration: The nine-month treatment period may not capture long-term effects of INI.
- Subjectivity: Assessments of pragmatic abilities were partly based on subjective observations and interviews.
- Compliance: Variability in treatment adherence (underdosing) may have influenced the results.
Using Intranasal Insulin for Cognition (Recommendations)
1. Consult with a Healthcare Professional
Before starting any treatment with intranasal insulin (INI), it is crucial to consult with a healthcare provider, preferably a neurologist or endocrinologist, who can assess the suitability of INI for your specific condition.
2. Eligibility & Screening
- Medical History Review: The healthcare provider will review your medical history, including any existing conditions such as diabetes, insulin resistance, or cognitive impairment.
- Baseline Assessments: Initial assessments may include cognitive tests, MRI scans, and blood tests to determine insulin levels and glucose metabolism.
3. Choosing the Right Device
- Intranasal Delivery Device: Use an approved intranasal delivery device, such as the ViaNase device, which is specifically designed to transport insulin effectively into the brain.
- Proper Usage: Ensure you are trained by a healthcare professional on the correct use of the device to maximize its effectiveness and minimize risks.
4. Dosage & Administration
- Starting Dosage: The typical starting dosage is 40 IU of human insulin (Novolin R) administered twice daily. However, your healthcare provider may adjust the dosage based on individual response and tolerance.
- Administration Technique: Follow these steps for safe administration:
- Preparation: Ensure the device is clean and filled with the correct insulin dosage.
- Positioning: Insert the device into one nostril while keeping your head slightly tilted back.
- Delivery: Activate the device to release the insulin into the nasal cavity, aiming towards the upper part of the nasal cavity.
- Repeat: Administer the dose in the other nostril if required.
- Cleaning: Clean the device after each use according to the manufacturer’s instructions.
5. Monitoring and Adjustments
- Regular Monitoring: Schedule regular follow-ups with your healthcare provider to monitor the effectiveness and any side effects of the treatment. This may include cognitive assessments, blood tests, and MRI scans.
- Adjustments: Based on the results and side effects, your provider may adjust the dosage or frequency of administration.
6. Potential Side Effects and Management
- Common Side Effects: These may include mild nasal irritation, headache, or transient dizziness.
- Serious Side Effects: Rarely, hypoglycemia (low blood sugar) or allergic reactions may occur. Seek immediate medical attention if you experience severe symptoms such as difficulty breathing, swelling, or severe hypoglycemia.
7. Lifestyle Considerations
- Diet & Exercise: Maintain a balanced diet and regular exercise routine to support overall brain health and complement the effects of INI treatment.
- Medication Interactions: Inform your healthcare provider about any other medications or supplements you are taking to avoid potential interactions.
Conclusion: Intranasal Insulin for MCI
The study demonstrated that extended intra-nasal insulin (INI) treatment significantly improves cognitive and pragmatic abilities in a patient with insulin-resistant early Mild Cognitive Impairment (MCI).
The patient, EJ, showed increased grey matter volume, reduced beta-amyloid levels, and enhanced performance on cognitive tests, alongside improvements in social engagement and procedural memory.
These findings suggest that INI can positively impact both brain structure and function, potentially slowing the progression of neurodegenerative diseases.
While the results are promising, further research with larger sample sizes and longer follow-up periods is needed to confirm the efficacy and safety of INI treatment for cognitive impairments.
References
- Study: Enhancing socio-communicative functions in an MCI patient with intra-nasal insulin: a case report (2024)
- Authors: Sara Schatz & Grace Rose Gutiérrez