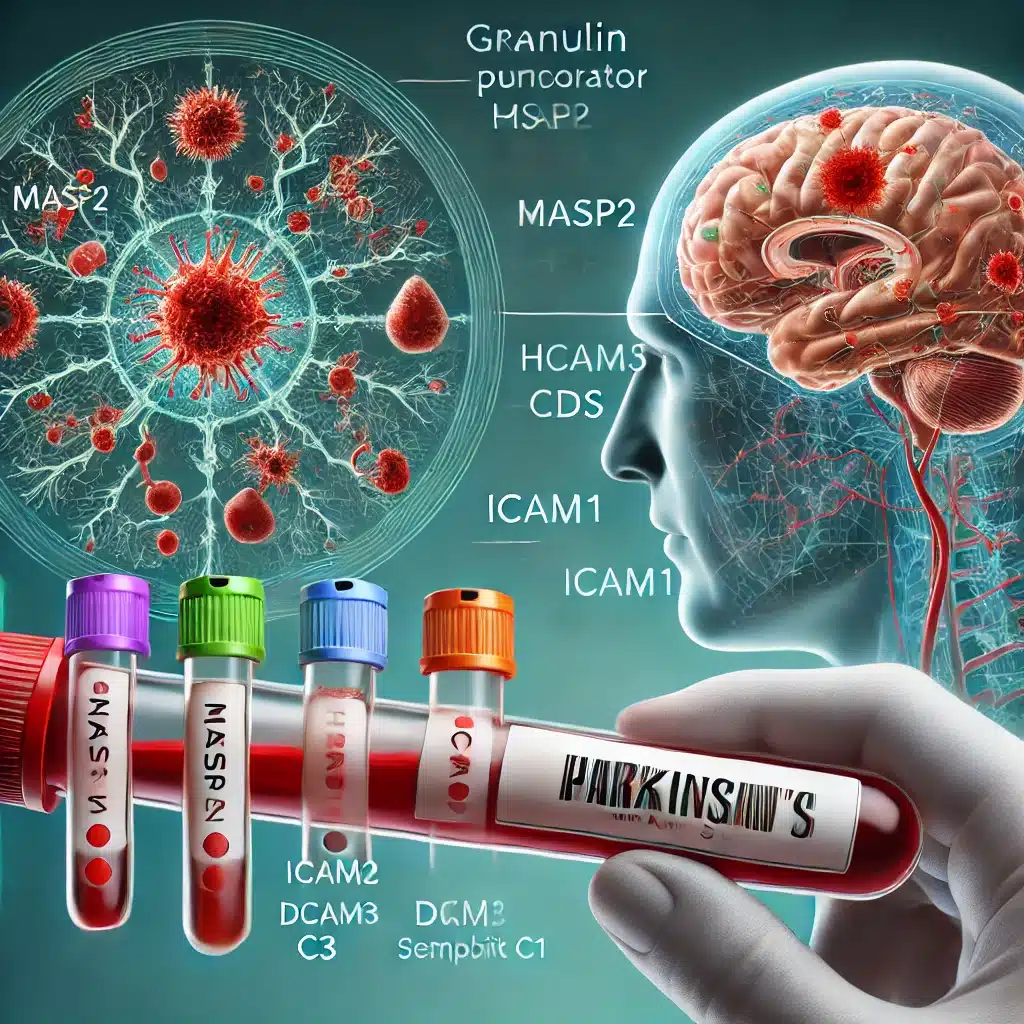
A study identified a panel of eight plasma biomarkers that can predict Parkinson’s disease up to seven years before motor symptoms appear.
Highlights:
- Biomarker Panel: The study validated a panel of 8 proteins, including Granulin precursor and Complement C3, using mass spectrometry, which can differentiate Parkinson’s patients from healthy controls and identify pre-motor individuals with REM sleep behavior disorder (iRBD).
- Prediction Accuracy: The machine-learning model based on these biomarkers could accurately classify all Parkinson’s patients and 79% of pre-motor individuals up to seven years before symptom onset.
- Inflammation Markers: Many of the identified biomarkers are associated with inflammatory pathways, suggesting inflammation as an early event in Parkinson’s pathology.
- Clinical Correlation: The expression levels of these biomarkers correlated with the severity of Parkinson’s symptoms, indicating their potential use in monitoring disease progression.
- Early Detection: This blood-based biomarker panel provides a non-invasive method for early detection, enabling at-risk individuals to be identified and possibly included in prevention trials.
Source: Nature (2024)
Parkinson’s Disease: Detection, Symptoms, Causes
Early Detection
Typical Detection Time
Parkinson’s disease (PD) is usually detected during the motor stage, which occurs several years after the initial onset of the disease.
Most patients are diagnosed when they start showing visible motor symptoms such as tremors and rigidity.
Challenges in Early Detection
Early, non-motor symptoms often go unnoticed or are misattributed to other conditions.
There is a significant delay between the onset of initial symptoms and a formal diagnosis, making early intervention difficult.
Signs & Symptoms
Motor Symptoms
- Tremors: Involuntary shaking, often starting in the hands or fingers.
- Bradykinesia: Slowness of movement, making everyday tasks difficult and time-consuming.
- Rigidity: Stiffness in the limbs and trunk, leading to decreased range of motion.
- Postural Instability: Problems with balance and coordination, increasing the risk of falls.
Non-Motor Symptoms
- REM Sleep Behavior Disorder (RBD): Acting out dreams during sleep, which can be an early sign of PD.
- Anosmia: Loss of the sense of smell, often preceding motor symptoms.
- Constipation: Gastrointestinal issues due to slowed autonomic functions.
- Depression and Anxiety: Common psychological symptoms that may appear before motor symptoms.
- Cognitive Impairment: Memory problems and slowed thinking can develop as the disease progresses.
Causes
Genetics
Mutations in specific genes, such as LRRK2, PARK7, and SNCA, have been linked to familial forms of Parkinson’s disease.
Genetic predispositions increase the risk but do not guarantee the development of the disease.
Environment
Exposure to certain pesticides and heavy metals has been associated with a higher risk of developing PD.
Rural living and well water consumption are also considered risk factors.
Pathophysiology
- Loss of Dopaminergic Neurons: PD is characterized by the degeneration of dopamine-producing neurons in the substantia nigra, a region of the brain involved in movement control.
- Lewy Bodies: Abnormal aggregates of protein, mainly alpha-synuclein, form inside neurons, disrupting normal cellular function and leading to cell death.
- Inflammation: Recent studies suggest that inflammation and immune system dysfunction play a crucial role in the early stages of PD.
Main Findings: Biomarkers to Detect Parkinsons Disease Years Before Onset (2024)
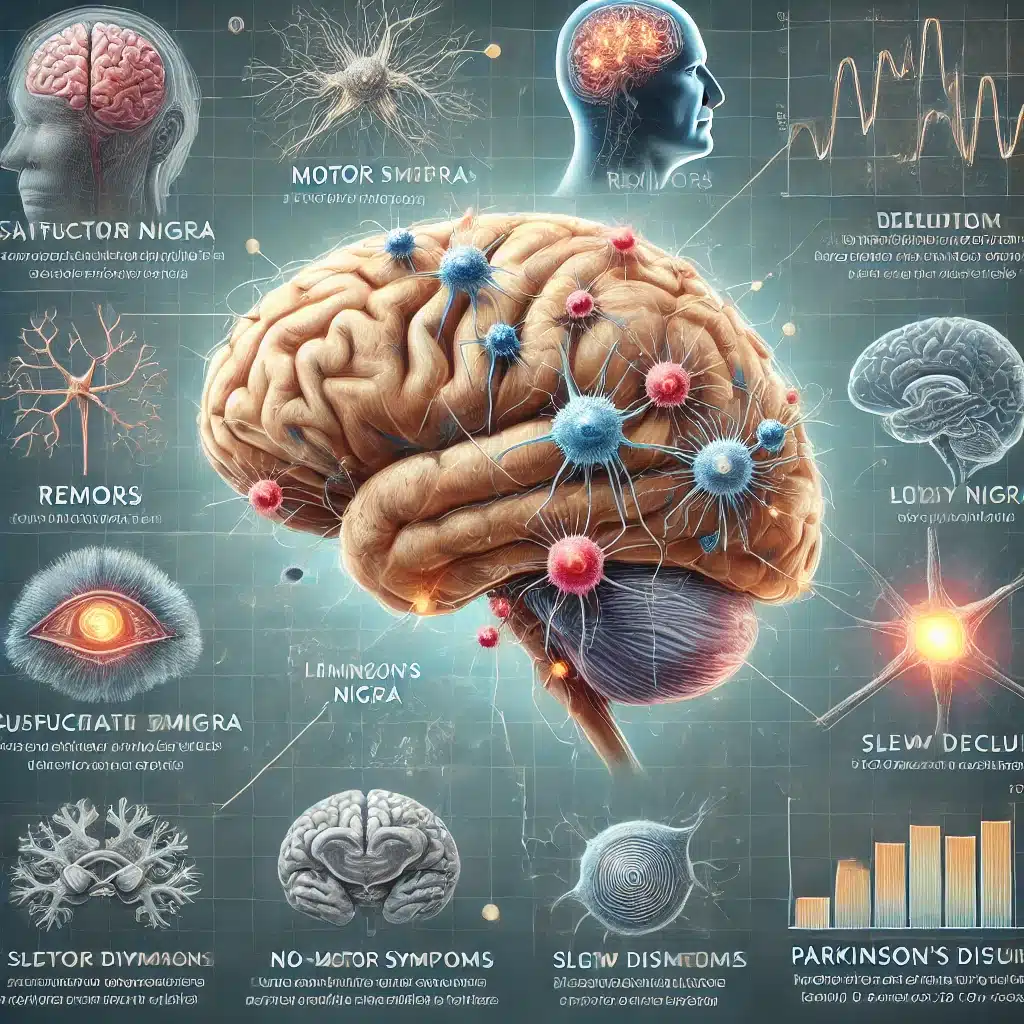
1. Early Detection of Parkinson’s Disease
The study discovered a panel of eight blood proteins that can predict Parkinson’s disease (PD) up to seven years before the onset of motor symptoms.
This is a significant breakthrough because early detection can potentially lead to early intervention and better management of the disease.
2. Biomarkers Identified
- Granulin precursor
- Mannan-binding-lectin-serine-peptidase-2 (MASP2)
- Endoplasmic-reticulum-chaperone-BiP (HSPA5)
- Prostaglandin-H2-D-isomerase (PTGDS)
- Intercellular-adhesion-molecule-1 (ICAM1)
- Complement C3
- Dickkopf-WNT-signaling-pathway-inhibitor-3 (DKK3)
- Plasma-protease-C1-inhibitor (SERPING1)
These proteins were measured using a targeted mass spectrometry assay.
3. High Prediction Accuracy
Using machine learning, the researchers developed a model that could:
- Accurately classify all patients with Parkinson’s disease from healthy individuals.
- Identify 79% of individuals in the pre-motor stage (those with isolated REM sleep behavior disorder) up to seven years before motor symptoms appeared.
4. Inflammation as a Key Factor
The study highlighted that many of these biomarkers are linked to inflammatory processes in the body.
This suggests that inflammation plays a crucial role early in the development of Parkinson’s disease, even before motor symptoms become apparent.
5. Correlation with Symptom Severity
The levels of these biomarkers were found to correlate with the severity of Parkinson’s symptoms. For example:
- Higher levels of Complement C3 and SERPING1 were associated with more severe motor symptoms.
- Lower levels of DKK3 and Granulin precursor were linked to worse cognitive performance and more advanced disease stages.
Study Basics: Plasma Biomarkers for Early Detection of Parkinsons (2024)
Participants
Participants:
99 recently diagnosed motor Parkinson’s patients.
Two pre-motor cohorts with isolated REM sleep behavior disorder (iRBD):
- Cohort 1: 18 individuals.
- Cohort 2: 54 individuals (longitudinally followed).
36 healthy controls.
Methods
Proteomic Analysis
A targeted multiplexed mass spectrometry assay was used to measure the levels of specific proteins in the blood samples.
The study initially identified 895 distinct proteins, of which 47 were differentially expressed between Parkinson’s patients and healthy controls.
Biomarker Panel Development
From the discovery phase, eight proteins were selected based on their significant differential expression.
These proteins were validated in an independent cohort using a high-throughput targeted proteomic assay.
Machine Learning Model
A machine-learning model was developed to classify and predict Parkinson’s disease based on the expression levels of the identified proteins.
The model achieved 100% accuracy in differentiating Parkinson’s patients from healthy controls and 79% accuracy in identifying pre-motor individuals.
Limitations
- Sample Size: The study had a relatively small sample size, especially in the pre-motor cohorts. Larger studies are needed to confirm these findings.
- Generalizability: The study participants were from specific cohorts with rigorous inclusion criteria. The findings may need to be validated in more diverse populations.
- Longitudinal Validation: Although the study included longitudinal data for some participants, longer follow-up periods are required to fully understand the predictive power of the biomarkers over time.
- Protein Correlation: The correlation between plasma proteins and cerebrospinal fluid proteins was poor, indicating that blood biomarkers might not fully reflect the pathological processes occurring in the brain.
- Technological & Practical Considerations: While mass spectrometry is highly accurate, it is also complex and may not be readily available in all clinical settings. Simplifying the test for widespread clinical use will be necessary.
(Related: Gut Bacteria Abnormalities in Parkinsons Disease)
Future Applications & Other Emerging Methods for Predicting Parkinson’s Disease
Potential for Clinical Use
Current Status
The newly identified blood-based biomarker panel holds promise for early detection of Parkinson’s disease (PD).
However, further validation in larger and more diverse cohorts is necessary before it can be implemented in clinical practice.
Advantages
- Non-Invasive: Unlike cerebrospinal fluid tests that require lumbar punctures, this blood test is less invasive and more convenient for patients.
- Early Detection: The ability to predict PD up to seven years before motor symptoms appear could revolutionize how the disease is managed, allowing for earlier interventions.
- Cost-Effectiveness: A blood test is generally more cost-effective and easier to administer on a large scale compared to more invasive diagnostic methods.
Challenges
- Validation & Standardization: Extensive validation in various clinical settings is required to ensure the test’s reliability and accuracy across different populations.
- Integration into Healthcare Systems: Healthcare providers will need to be trained on how to use and interpret the results of this new test.
- Regulatory Approval: Regulatory bodies will need to review and approve the test for clinical use, which can be a lengthy process.
Other Emerging Methods for Parkinson’s Disease Prediction
Alpha-Synuclein Seed Amplification Assay (SAA)
This method detects misfolded alpha-synuclein protein aggregates in cerebrospinal fluid.
- Advantages: High sensitivity and specificity for PD, especially in the prodromal stages.
- Limitations: Requires lumbar punctures, making it less suitable for widespread screening.
Neuroimaging Techniques
Advanced imaging techniques, such as PET and MRI scans, can detect early changes in the brain associated with PD.
- Advantages: Provides detailed information about brain structure and function.
- Limitations: Expensive, time-consuming, and not always accessible for routine screening.
Genetic Testing
Identifies genetic mutations associated with a higher risk of developing PD.
- Advantages: Useful for individuals with a family history of PD.
- Limitations: Genetic predisposition does not guarantee the development of the disease, making it less predictive for the general population.
Other Blood Biomarkers
Research is ongoing to identify other blood-based biomarkers that could indicate early PD.
- Examples: Neurofilament light chain (NfL) and other proteins involved in neuroinflammation and neuronal damage.
- Advantages: Potential for non-invasive and cost-effective early detection.
- Limitations: Many biomarkers are still in the research phase and require further validation.
(Related: Retinal Imaging Detects Parkinsons Disease Up to 7 Years in Advance)
Multimodal Approach
Combining different methods, such as the new biomarker panel, genetic testing, and neuroimaging, could provide a more comprehensive approach to early detection and diagnosis of PD.
Conclusion: Plasma Biomarkers in Parkinsons Disease
References
- Study: Plasma proteomics identify biomarkers predicting Parkinson’s disease up to 7 years before symptom onset (2024)
- Authors: Jenny Hällqvist et al.