High-intensity exercise (HIIE) leads to significant changes in brain activity, specifically in regions linked to dopaminergic and μ-opioidergic neurotransmission, which are associated with a mood boost.
Highlights:
- Positive Mood Effects: Both low- and high-intensity exercise increased positive affect, as measured by the PANAS Positive Affect scale.
- Brain Activity Changes: HIIE resulted in significant decreases in brain activity in regions like the precuneus, orbitofrontal cortex, and thalamus, while increasing activity in the hypothalamus, pituitary, and periaqueductal gray.
- Neurotransmitter Involvement: Spatial correlations indicated that HIIE-induced brain activity changes were associated with dopaminergic (D2 receptors) and μ-opioidergic (Mu receptors) neurotransmission within reward-related brain networks.
- Endocannabinoid System: There was weaker evidence suggesting involvement of the endocannabinoid system, specifically the CB1 receptors, in response to HIIE.
Source: Frontiers in Neuroimaging (2024)
Major Findings: High Intensity Interval Training Exercise & Brain Activity (2024)
1. Mood Boost
The study observed that both low-intensity interval exercise (LIIE) and high-intensity interval exercise (HIIE) significantly improved participants’ positive mood.
This was measured using the Positive and Negative Affect Schedule (PANAS) scores.
After engaging in both LIIE and HIIE, participants reported higher levels of positive affect compared to before the exercises.
This suggests that physical activity, regardless of intensity, can enhance mood and promote feelings of well-being.
2. Brain Activity Changes
High-intensity exercise (HIIE) led to significant changes in brain activity, detectable through resting-state functional MRI (rs-fMRI).
Specifically, decreases in fractional amplitude of low-frequency fluctuations (zfALFF) were found in regions such as the precuneus, orbitofrontal cortex, inferior temporal gyrus, thalamus, and cerebellum.
In contrast, zfALFF increases were noted in the hypothalamus, pituitary, and periaqueductal gray.
These findings indicate that HIIE can alter neural activity in specific brain regions associated with various functions, including reward processing and homeostatic regulation.
3. Neurotransmitter Involvement
The study found that changes in brain activity due to HIIE were correlated with the distribution of certain neurotransmitters, particularly within the brain’s reward networks.
Dopaminergic System: The spatial patterns of zfALFF changes were positively correlated with the distribution of D2 dopamine receptors. This suggests that dopamine, which is crucial for motivation and reward, plays a significant role in how the brain responds to high-intensity exercise.
μ-Opioidergic System: Similarly, there was a positive correlation with μ-opioid receptors, indicating that the brain’s natural opioid system, which is associated with pleasure and pain relief, is also actively involved during high-intensity exercise.
4. Endocannabinoid System
There was weaker but noteworthy evidence suggesting the involvement of the endocannabinoid system in response to high-intensity exercise.
The spatial correlation with CB1 receptors, although not as strong as for dopamine and opioid receptors, points to a potential role of endocannabinoids in mediating the brain’s response to strenuous physical activity.
The endocannabinoid system is known for its role in regulating mood, pain, and reward, and its engagement during exercise might contribute to the overall positive affect and pain relief experienced post-exercise.
5. Resting-State fMRI & Neurotransmitter Maps
The study employed an innovative approach by combining resting-state fMRI with neurotransmitter distribution maps to infer changes in neurotransmission indirectly.
This method allowed the researchers to examine multiple neurotransmitter systems simultaneously without the need for invasive procedures like PET scans.
The findings support the utility of this non-invasive technique in providing insights into the complex neurobiological effects of acute exercise, paving the way for future research that can further explore these mechanisms in different populations and settings.
Study Overview: Exercise Intensity vs. Brain Activity & Neurotransmission (2024)
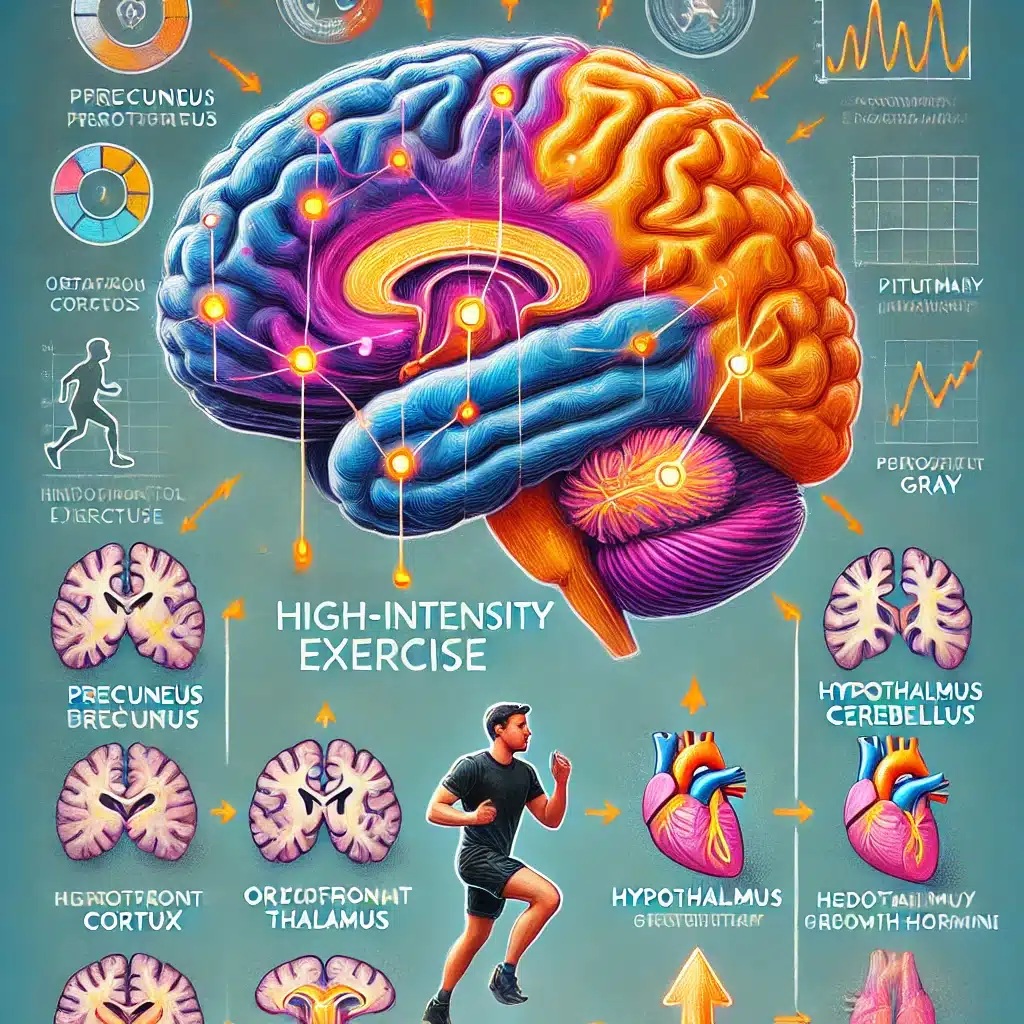
The study evaluated how different intensities of acute exercise affect brain activity and neurotransmitter systems, particularly those related to mood and reward processes.
Researchers focused on changes in fractional amplitude of low-frequency fluctuations (zfALFF) in resting-state functional MRI (rs-fMRI) and their spatial correlations with neurotransmitter maps.
Sample
- Participants: 20 young, healthy, well-trained male athletes (20-35 y/o)
- Selection Criteria: Participants with a relative maximum oxygen uptake (relVO2max) of at least 55 mL/min/kg were included to ensure high fitness levels.
Methods
- Experimental Design: Within-subject design with three different conditions: low-intensity interval exercise (LIIE), high-intensity interval exercise (HIIE), and a control condition. Each condition was performed on separate days in a randomized order.
- LIIE: 4 x 4-minute intervals at 100% of the first rise in lactate concentration, with 3-minute active recovery.
- HIIE: 4 x 4-minute intervals at 110% of Dmax (the highest workload achieved in the performance test), with 3-minute active recovery.
- Control: Sitting on the bicycle ergometer without load for the same duration.
- Mood Assessment: Positive and Negative Affect Schedule (PANAS) scores were recorded before and after each exercise session.
- Brain Activity: Resting-state fMRI scans were taken before and after each exercise session to measure zfALFF.
- Data Analysis: Repeated measures ANOVAs were used to analyze zfALFF changes, and spatial correlations with neurotransmitter maps were performed using the JuSpace toolbox.
Limitations
- Sample Size: The study included only 20 participants, which may limit the generalizability of the findings.
- Sex Restriction: Only male athletes were included to minimize hormonal fluctuation variance, limiting the applicability of results to females.
- Exercise Mode: The study focused on cycling, so findings may not directly apply to other forms of exercise.
- Indirect Measurement: The use of spatial correlations with neurotransmitter maps provides indirect evidence and cannot confirm causal relationships.
- Time of Day: While examinations were controlled for individual participants, measurements were conducted during normal working hours, which might introduce variability related to circadian rhythms.
Specific Brain Changes from High-Intensity Exercise

High-intensity interval exercise (HIIE) led to significant changes in brain activity as detected by resting-state functional MRI (rs-fMRI) through fractional amplitude of low-frequency fluctuations (zfALFF):
Decreases in zfALFF
Precuneus: This region is involved in self-processing and consciousness. A decrease in activity here may indicate a shift in focus from internal thoughts to external physical activity.
Orbitofrontal Cortex: Linked to decision-making and reward processing. Reduced activity might reflect a transient downregulation as the brain reallocates resources during intense physical exertion.
Thalamus: Acts as a relay center for sensory and motor signals. Decreased activity could indicate a temporary reduction in sensory processing to prioritize motor control during exercise.
Cerebellum: Essential for motor control and coordination. Decreased zfALFF here may reflect efficiency improvements in motor function and coordination during high-intensity exercise.
Increases in zfALFF
Hypothalamus: This region regulates bodily functions such as temperature, hunger, and the stress response. Increased activity indicates heightened regulation of these essential functions during intense physical exertion.
Pituitary Gland: Known as the “master gland,” it controls various hormonal responses. Increased activity suggests a robust endocrine response to manage the physical stress of high-intensity exercise.
Periaqueductal Gray (PAG): Involved in pain modulation and antinociception. Increased activity here is beneficial as it can help in pain suppression, allowing sustained physical performance.
What were the effects of these brain changes?
Mood Improvement
Positive Mood: Both LIIE and HIIE significantly increased positive mood, with participants reporting higher levels of positive affect after exercise. This is beneficial for mental health, reducing stress, anxiety, and depression.
Pain Management
Increased PAG Activity: Enhanced activity in the PAG region suggests better pain modulation, which can help athletes endure higher levels of physical exertion without discomfort. This is advantageous for improving athletic performance and sustaining longer periods of exercise.
Hormonal Regulation
Hypothalamus & Pituitary Activation: The increase in activity of these regions indicates a robust hormonal response, which helps in maintaining homeostasis during intense physical activity. This includes the regulation of cortisol (stress hormone) and other critical hormones, aiding in recovery and adaptation to physical stress.
Enhanced Reward Processing
Orbitofrontal Cortex & Dopaminergic System: Although there was a decrease in zfALFF in the orbitofrontal cortex, the spatial correlation with dopamine receptors in the reward network suggests an enhanced reward response post-exercise. This can lead to greater motivation and pleasure from physical activity, encouraging regular exercise habits.
Mechanisms of Brain Changes via High-Intensity Exercise
1. Neurotransmitter Release
High-intensity exercise triggers the release of various neurotransmitters that play crucial roles in mood regulation, pain management, and overall brain function.
Dopamine
- Mechanism: Dopamine is released in response to physical activity, particularly in the brain’s reward pathways (ventral tegmental area and nucleus accumbens).
- Reason: The increase in dopamine promotes feelings of pleasure and reward, enhancing mood and motivation. This release is particularly robust during high-intensity exercise, which is demanding and thus elicits a stronger dopaminergic response to reinforce the behavior.
Endorphins
- Mechanism: Endorphins are endogenous opioids released by the hypothalamus and pituitary gland during exercise.
- Reason: They bind to opioid receptors in the brain, leading to analgesic (pain-relieving) effects and feelings of euphoria, commonly known as the “runner’s high.” This helps athletes manage pain and discomfort during strenuous exercise.
Endocannabinoids
- Mechanism: Exercise stimulates the production of endocannabinoids, such as anandamide, which bind to cannabinoid receptors (CB1 and CB2) in the brain.
- Reason: These compounds play a role in pain relief, mood enhancement, and the regulation of stress and anxiety, contributing to the positive affect experienced after exercise.
2. Hormonal Responses
High-intensity exercise activates the hypothalamic-pituitary-adrenal (HPA) axis, leading to the release of various hormones.
Cortisol
- Mechanism: The hypothalamus releases corticotropin-releasing hormone (CRH), which stimulates the pituitary gland to produce adrenocorticotropic hormone (ACTH). ACTH then triggers the adrenal glands to release cortisol.
- Reason: Cortisol helps the body manage stress by regulating energy production, immune response, and metabolic processes. This hormonal response ensures that the body can handle the physical stress of intense exercise and recover effectively.
Growth Hormone
- Mechanism: High-intensity exercise stimulates the release of growth hormone (GH) from the pituitary gland.
- Reason: GH supports tissue repair, muscle growth, and overall recovery after intense physical activity. It also plays a role in metabolic regulation and the mobilization of fat for energy.
3. Increased Blood Flow & Oxygenation
Exercise, especially at high intensity, significantly increases cerebral blood flow (CBF) and oxygen delivery to the brain.
- Mechanism: During high-intensity exercise, cardiac output increases, and blood vessels in the brain dilate, enhancing blood flow. This process is mediated by nitric oxide and other vasodilators.
- Reason: Increased blood flow delivers more oxygen and nutrients to the brain, supporting heightened neural activity and the maintenance of optimal brain function. It also aids in the removal of metabolic waste products.
4. Neuroplasticity & Synaptic Adaptation
Exercise induces neuroplastic changes, enhancing the brain’s ability to adapt and reorganize.
- Mechanism: High-intensity exercise promotes the release of brain-derived neurotrophic factor (BDNF), a protein that supports the growth, survival, and differentiation of neurons and synapses.
- Reason: BDNF enhances neuroplasticity, which improves cognitive function, learning, and memory. Regular high-intensity exercise can lead to long-term structural changes in the brain, fostering resilience against neurological disorders and cognitive decline.
5. Modulation of Brain Networks
Exercise affects the functional connectivity between different brain regions.
- Mechanism: High-intensity exercise alters the synchronization of neural activity within and between various brain networks, such as the default mode network (DMN), executive control network (ECN), and sensorimotor network.
- Reason: These changes enhance the brain’s efficiency in processing information, regulating emotions, and coordinating motor functions. Improved connectivity in these networks supports better cognitive performance and emotional regulation.
Conclusion: High-Intensity Interval Training (HIIT) & the Brain
This study demonstrates that high-intensity interval exercise (HIIE) leads to significant changes in brain activity, particularly in regions associated with reward processing, pain modulation, and homeostatic regulation.
These changes are driven by complex mechanisms involving the release of neurotransmitters such as dopamine and endorphins, increased hormonal activity, enhanced blood flow, and neuroplastic adaptations.
The observed alterations in zfALFF indicate that HIIE positively impacts mood, pain tolerance, and cognitive function, highlighting the importance of physical activity for mental and physical health.
Future research should further explore these mechanisms in larger and more diverse populations to deepen our understanding of exercise’s neurobiological effects and its potential therapeutic applications.
References
- Study: Fractional amplitude of low-frequency fluctuations associated with μ-opioid and dopamine receptor distributions in the central nervous system after high-intensity exercise bouts (2024)
- Authors: Henning Boecker et al.